EQuIS Data Qualification Module
What is data validation?
Independent data validation of analytical laboratory results provides confidence in data for decision making associated with site characterization, remedial responses, and risk assessment. Data validation is an analyte- and sample-specific process that evaluates data to determine the analytical quality of a specific data set. Data validation includes a review of data for completeness of the data deliverables, correctness of the reported analytical results, compliance with the requirements of the method and project, and usability of the reported analytical results.
What is EQuIS DQM?
The EQuIS Data Qualification Module (DQM) is an automated data validation tool within EQuIS Professional and is licensed in the EQuIS Professional PremierD and PremierDG Libraries. DQM supports data validation, ensuring sample data meet established data quality criteria. DQM includes checks to assess data for holding times, blank contamination, surrogate recoveries, precision, accuracy, reporting limits, and more. DQM creates data validation events and event processing allows data qualifiers to be applied in batch. These events can be saved and re-opened as needed. The DQM qualification events are used to correct and modify reported laboratory data. Data quality reports are available directly in DQM, from the Reports in Professional, and via EQuIS Information Agents (EIAs) in Enterprise.

Data Checks Library
DQM is organized around data quality checks that can be selected to run against a data set. Each check has one or more rules that are applied to each result record in the data set to determine if a data qualifier and/or reason code should be applied to that record. Each rule has its own qualifier, but more than one rule can have the same qualifier. Each check also has a set of parameters, which allows DQM customization without changing any code.
DQM users may select existing data checks, configure existing checks, or build new data checks. DQM can add and modify data quality checks within the provided framework to meet client requirements and enable project-specific rules, quality assurance/quality control (QA/QC) limits, and data qualifiers. DQM allows data comparisons to configured or calculated criteria.
DQM Data Checks
• Holding Times
• Blank Contamination (Field, Trip, Lab)
• Estimated Results < Quantitation Limit
• Percent Solids
• Repeated Results
• Result Outliers (exceed user-defined numeric value)
• Reporting Detection Limit
Accuracy
• Surrogate Recoveries
• LCS/LCSD Recoveries
• MS/MSD Recoveries
Precision – Relative Percent Difference (RPD) between:
• LCS/LCSD recoveries
• MS/MSD recoveries
• Field Duplicate samples
• Laboratory Duplicate Samples
Radio Chemistry
• Estimated Results
• Relative Error Ratio
• Tracer Recovery
Advantages
DQM is Configurable
Every project has its own data quality objectives and DQM is configurable to meet project needs!
• Select checks to be run by DQM or customize your own
• Select quality control limits to be used by DQM for the checks
• Select qualifiers and related remarks to be applied by DQM
• Select the ranking of the qualifiers to be applied by DQM
• Select reason codes to be applied by DQM
• Select the ranking of the reason codes to be applied by DQM
Once configured, the settings can be saved and reused. Copy an existing configuration to leverage time savings for new project setup.
DQM is Flexible
Sample grouping reported by the laboratory may not be the ideal grouping for data validation. DQM allows users to easily select the samples to run in DQM. Select samples from an individual field sample delivery group, a chain-of-custody record, or analytical batch. Add associated samples to your selection.
DQM is Interactive
Once DQM is run on a set of data, review the DQM output using filters by the check, results, and samples to Validate and Qualify the DQM Event. Qualifiers, remarks, and reason codes assigned to the data by DQM can be changed to account for additional project-specific criteria by filtering on the exceptions report, the results, or the samples. A history of any changes to qualifiers will be maintained to allow for auditing. Review re-analyzed and re-extracted data and perform a selection of the “best of results”. Set which results should be “reportable”. Re-open DQM Events to process one check at a time, to add qualifiers based upon full validation, or for a secondary peer review process.
DQM is Efficient
Save time and money by using DQM to perform data validation. For large sites with routine regulatory reporting requirements, configure the project DQM QAPP once and then re-use for each new sampling event. Use out-of-the-box reports or configure your own to facilitate reporting.
DQM Workflow

Select QAPP and Checks
Qualification rules for a project Quality Assurance Project Plan (QAPP) are tailored by setting up a corresponding DQM QAPP that contains the checks, rules, and parameters needed to perform data validation in DQM.


Select Data Set
Easily select analytical results requiring qualification in DQM by Chains of Custody (COCs), Field Sample Delivery Groups (SDGs), Lab SDGs, Test Batch IDs, Task Codes, and/or Sample Codes.
Start Event
Each data validation effort is performed as a distinct event in DQM. A DQM event is a review of analytical results dataset(s) that are run through the automated checks for the selected DQM QAPP.
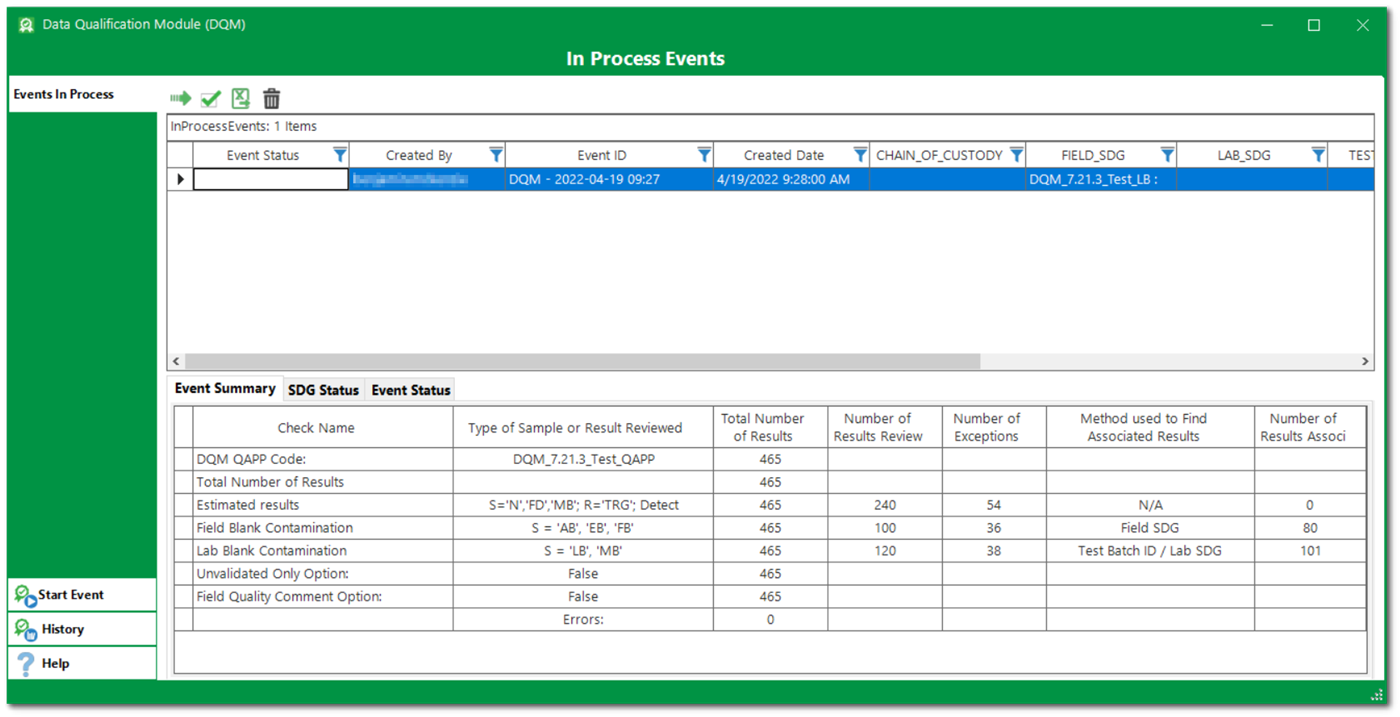

Review and Modify Suggested Qualifiers, Remarks, and Reason Codes
Data validators then exercise professional judgement to review the DQM generated flags by check, sample, or other filters. DQM events can be saved and re-opened as needed.
Save Qualifiers to EQuIS
Save the validated results, data qualifiers, remarks, and reason codes directly to the EQuIS database. No need to re-load an EDD. Additionally, users can make selections for which field in the database to save the qualifiers, whether to mark the data as validated, and if data will be considered “not-reportable” for reporting purposes.

Generate Summary Report(s)
DQM makes data reporting easy. Export summary information, exceptions by check, and all analytical results and associated qualifiers to a Microsoft Excel spreadsheet. The DQM Event Report can also be run in Professional or Enterprise. Leverage the automated reporting functionality of EQuIS Information Agents (EIAs) in EQuIS Enterprise and deliver the DQM Exceptions Report by EDD report to the validator’s inbox for review anytime new data is received.
Interested in Purchasing DQM?
Contact our Sales Team
Solutions
Want to Learn More?
Explore EQuIS Environmental Data Management Workflows
Contact Us
125 S. Alcaniz St., Suite 2
Pensacola, FL 32502
800.649.8855
info@earthsoft.com
Subscribe to Our Mailing List
Stay informed about our upcoming events, news,
and more.
